How Does The Energy Of A System Change During A Phase Change?
How does the energy of a system change during a phase change?. The increase in internal energy due to compression happens before the phase transition. In the case of freezing energy is subtracted as the molecules bond to one another. During the actual transition the gas must be able to transfer energy heat to the environment.
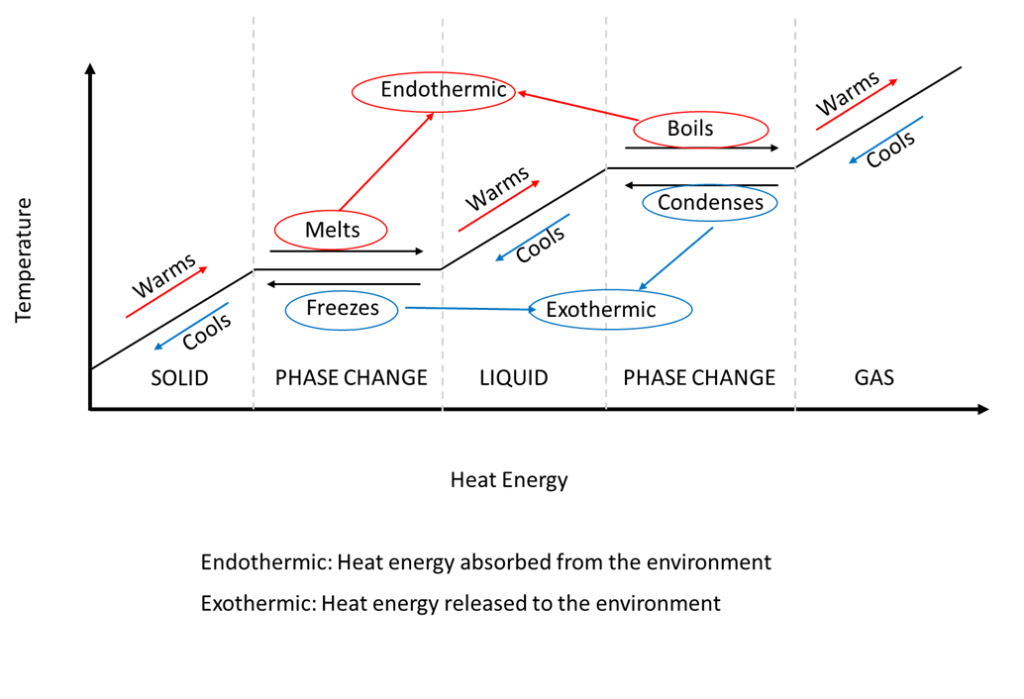
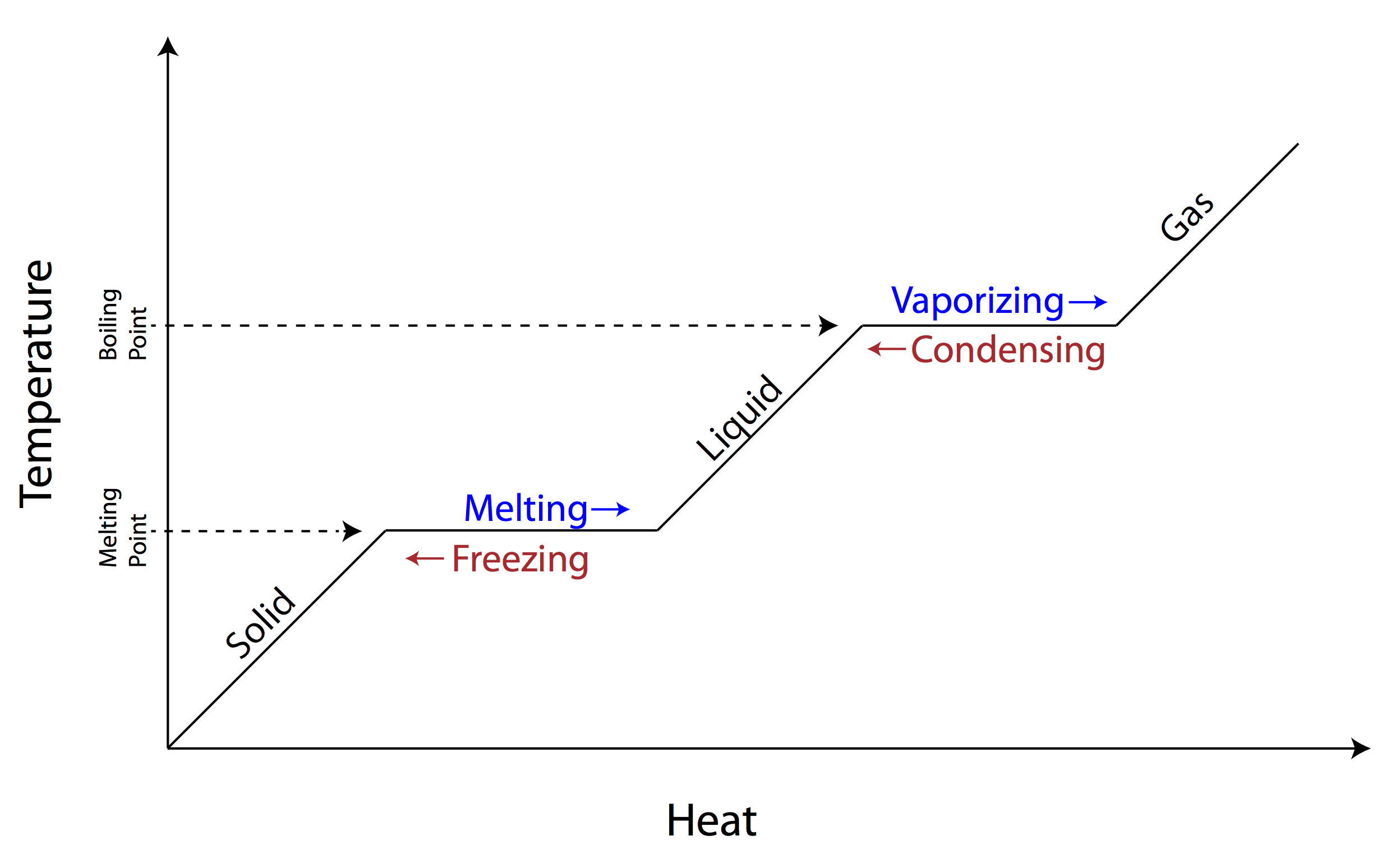
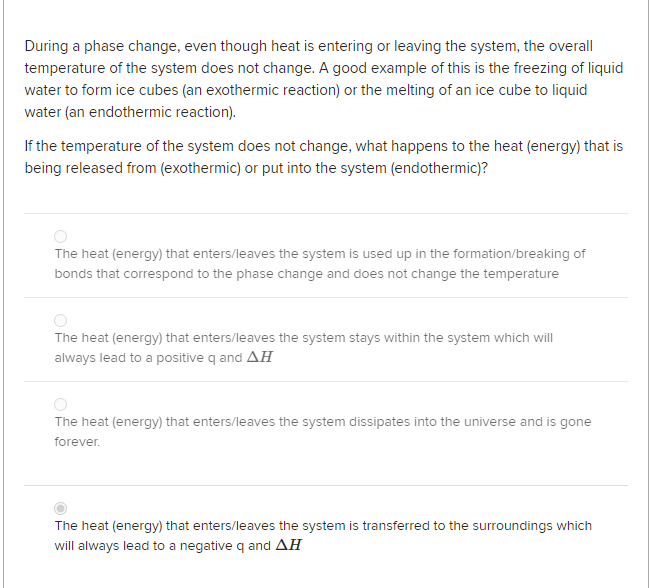
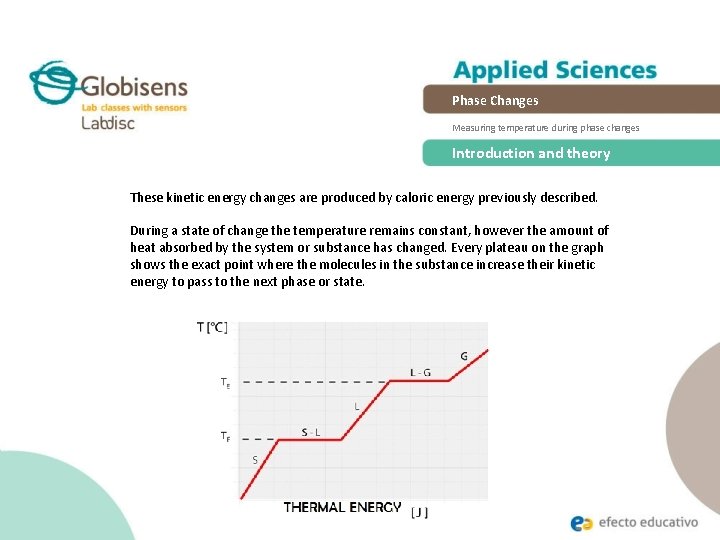
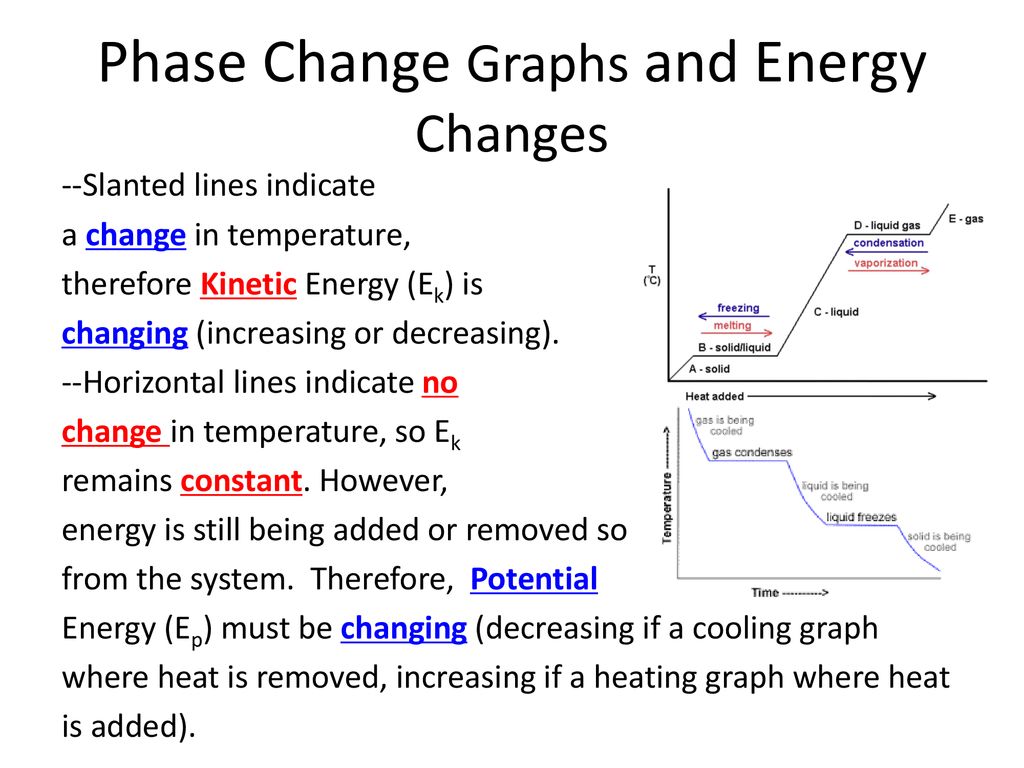
This energy is not used to increase the kinetic energy of the particles does not increase the temperature but to change the structure of matter. The increase in pressure brings the gas at the right conditions for molecular interactions to become strong enough for a condensed phase. During this process the temperature of the system will stay constant as heat is added.
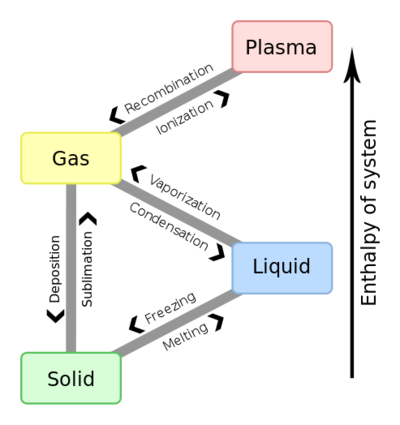
During a phase change two or more phases coexist in dynamic equilibrium. During a phase change the heat added PE increases or released PE decreases will allow the molecules to move apart or come together. In physical changes energy is used to change the state of matter.
In chemical changes energy is released when there is decomposition of a substance and absorbed while forming a new substance. They are changes in bonding energy between the molecules. If heat is coming into a substance during a phase change then this energy is used to.
These changes occur when sufficient energy is supplied to the system or a sufficient amount is lost and also occur when the pressure on the system is changed. During phase change there will be a change in latent energy which results in change in internal energy. For example converting a liquid in which the molecules are close together to a gas in which the molecules are on average far apart requires an input of energy heat to give the molecules enough kinetic energy to allow them to overcome the intermolecular attractive forces.
Work done in the process is given by W P ext ΔV. Removing heat from a substance changes a gas to a liquid or a liquid to a solid. The average kinetic energy stays constant overall the lost energy is transformed into potential energy and released back into the system as heat which becomes vibrational kinetic energy even though the particles slow down.
The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules. During a phase change internal energy of a system will change because energy must be added or subtracted depending on direction of change from the system in.
Heat absorbed causes the molecules to move farther apart by overcoming the intermolecular forces of attraction.
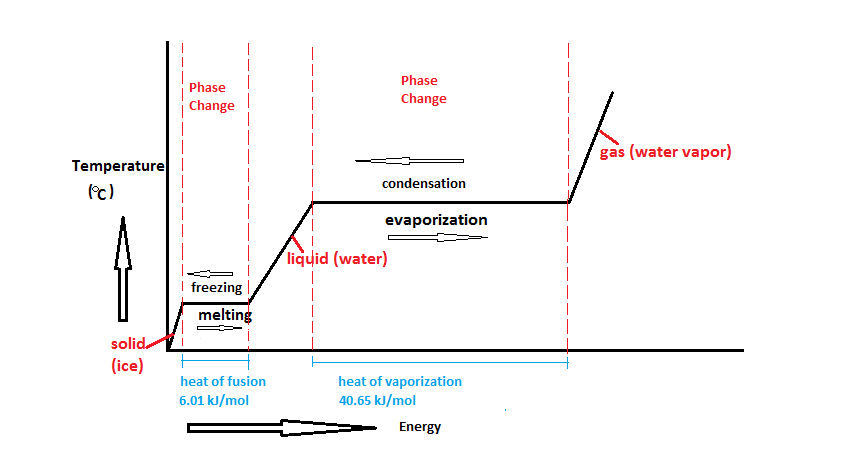
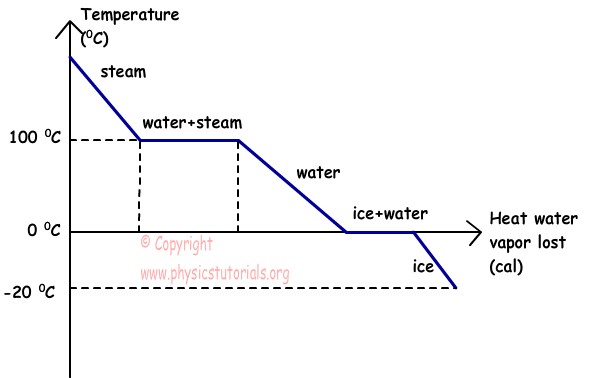
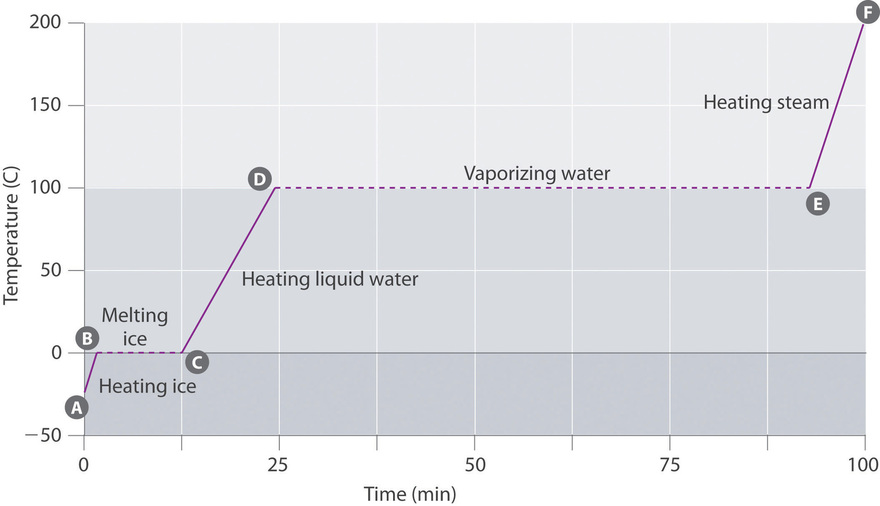
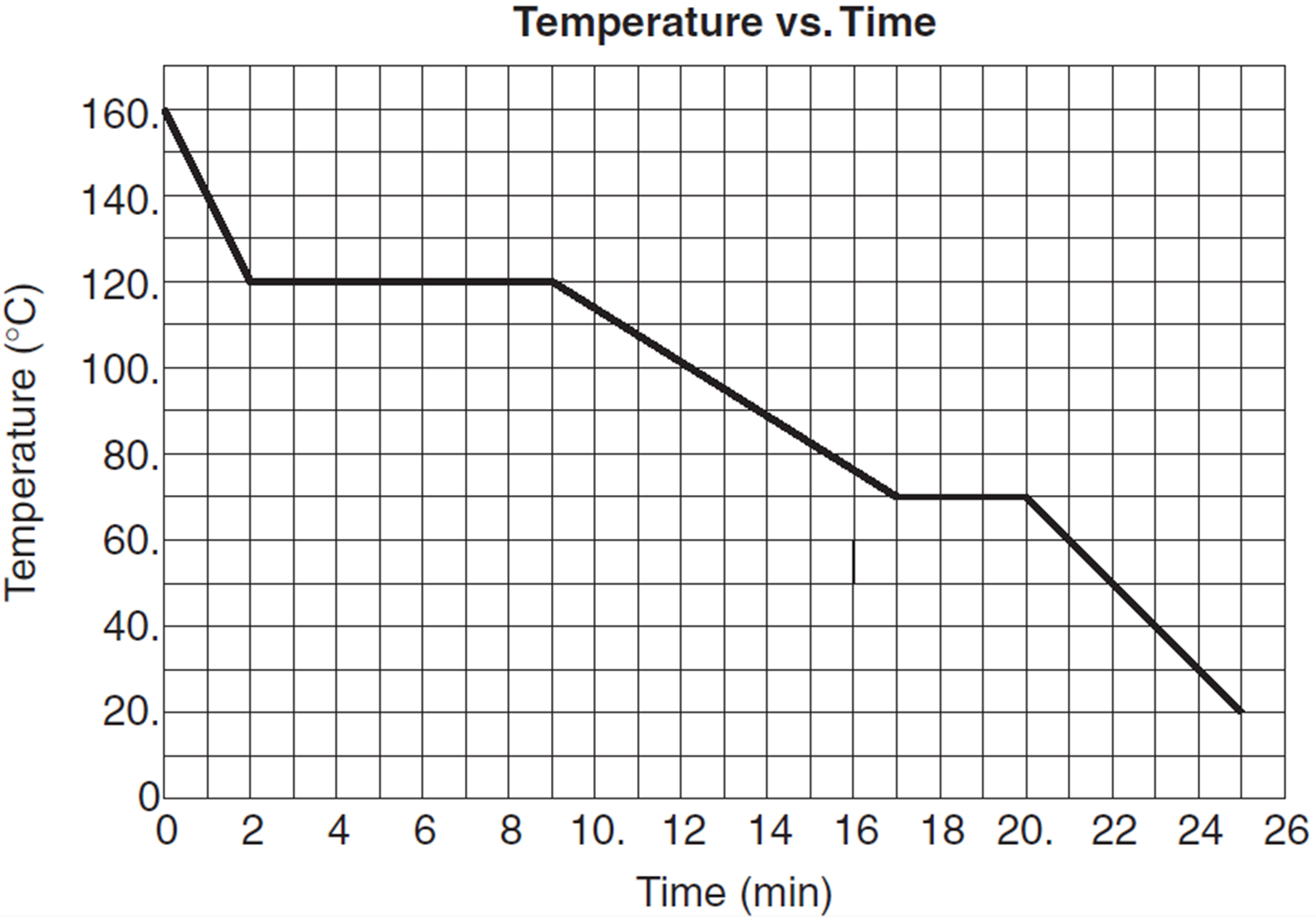
Heat going into a substance changes it from a solid to a liquid or a liquid to a gas. These energy exchanges are not changes in kinetic energy. However their temperature and thus average kinetic energy does not change during the freezing. The energy that is changing during a phase change is potential energy. During a phase change the heat added PE increases or released PE decreases will allow the molecules to move apart or come together. The hot liquid water returns to the boiler. A phase change is when matter changes to from one state solid liquid gas plasma to another. If heat is coming into a substance during a phase change then this energy is used to break the bonds between the molecules of the substance. The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules.
They are changes in bonding energy between the molecules. During the actual transition the gas must be able to transfer energy heat to the environment. During a phase change the temperature of a substance remains the same and any heat added to or subtracted from the system goes into changing phases. In the case of freezing energy is subtracted as the molecules bond to one another. These energy exchanges are not changes in kinetic energy. These energy exchanges are not changes in kinetic energy. During a phase change the heat added PE increases or released PE decreases will allow the molecules to move apart or come together.








/phase-changes-56a12ddd3df78cf772682e07.png)












/phase-changes-56a12ddd3df78cf772682e07.png)

Post a Comment for "How Does The Energy Of A System Change During A Phase Change?"